
Fusion is the combination of nuclei to form larger elements
Fission is the splitting of large elements into smaller ones
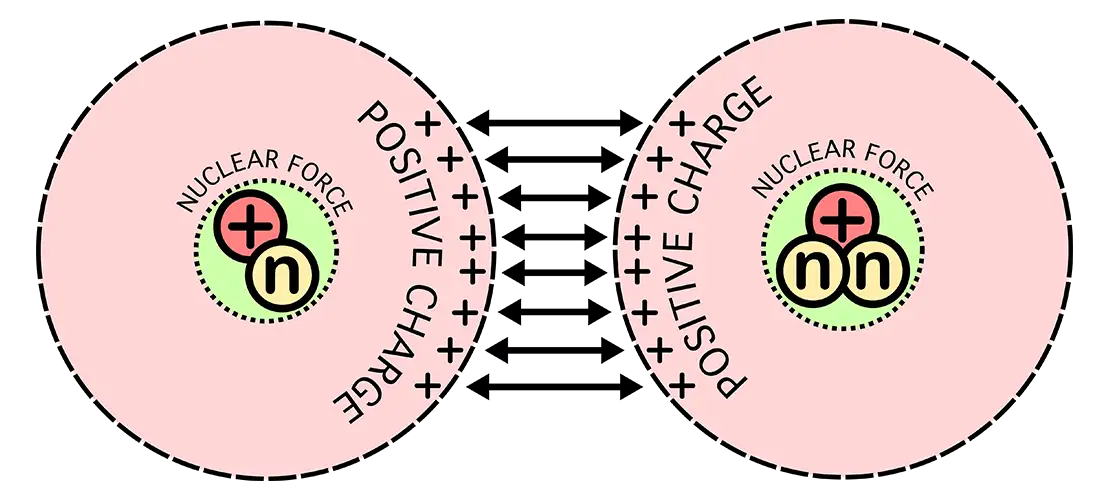
In order for fusion to occur, an electrostatic force called the coulomb barrier has to be overcome. Think of trying to push two repulsive sides of a magnet together.
A substantial amount of kinetic energy is required in order to overcome this repulsion. In the sun, this is achieved through high temperatures and immense gravity.
The sun achieves fusion at 15 million °C through a phenomena called quantum tunneling.
A nuclei behaves as both a particle and a wave on the quantum scale. It has a small probability to pass through a thin barrier.
The high concentration of fuel due to the sun's gravity allows for a suitable amount of reactions to sustain fusion.
On Earth we can not use gravity to exploit the quantum tunneling phenomena to the extent the sun is able to.
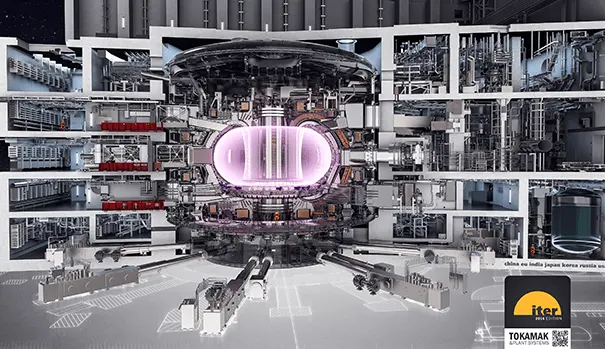
Instead we rely on turning up the heat to 150 million °C and using strong magnetic fields to manipulate the nuclei in a plasma state.
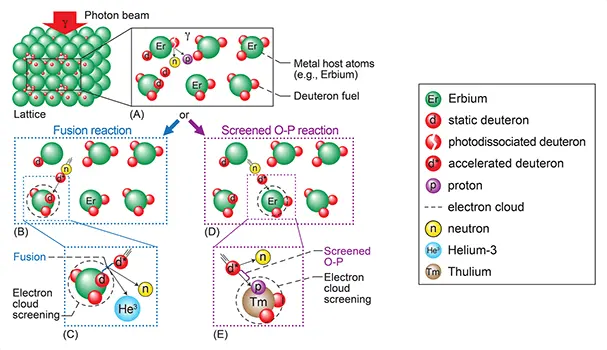
Or through novel methods such as Bremsstrahlung-irradiating deuterated metals. This method works through reducing the coulomb barrier by masking the fusion fuel in a metal lattice.
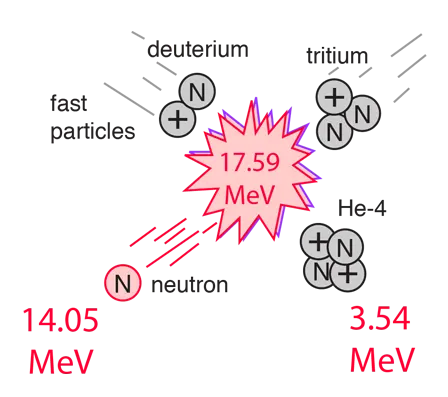
The D-T reaction occurs when a deuterium and tritium atom fuse together. It is regarded as the prominent reaction due to its low energy input and high output.
Fusion reactions release approximately 4 million times as much energy per unit mass than chemical reactions such as burning coal. Annually, a power plant would require 3 million tons of coal or simply 550 lb of D-T fuel.
It is estimated that 66 tons of D-T fuel is needed to meet the US annual energy consumption.
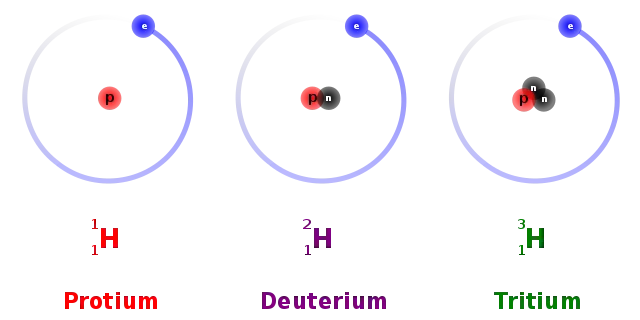
Deuterium, the hydrogen component of heavy water, is formed by the addition of a neutron.
Heavy water represents 0.015% of all ocean surface water which is over 10 trillion tons of deuterium.
This amount could meet the current US annual energy consumption for upwards of 300 billion years, the epitome of sustainability.
In order to obtain deuterium, heavy water must be extracted from oceanic water.
This can be achieved by the distillation of water, distillation of liquid H2, electrolysis, laser isotope separation, water-hydrogen sulphide exchange, ammonia-hydrogen catalyst exchange, aminomethanehydrogen catalyst exchange, or water-hydrogen catalyst exchange.
But water-hydrogen sulphide exchange (Girdler-Sulphide) is the most economical method for the quantity of deuterium needed.
This process was well established in Canada between 1980 and 1997 with 4 G-S plants, each producing 500 tons of heavy water annually (100 tons of deuterium).
The resulting heavy water must undergo electrolysis to create oxygen and deuterium gas.
Tritium is a hydrogen isotope that is formed by the addition of a neutron to deuterium.
Tritium is unstable and decays into helium-3 with a half-life of 12 years, which makes it rare in nature.
The tritium atom is radioactive but it can not penetrate more than 6 mm of air, most surfaces, or the outermost layer of skin.
The lithium-6 isotope, which accounts for 8% of all available lithium, when struck by energized neutrons ejects tritium and helium-4 atoms
It is estimated that first-generation reactors will be able to produce 150 grams of tritium per gigawatt per day by lining the inside of the reactor walls with lithium-6
However, the known worldwide land-based lithium ore reserve will be depleted by 2080 if consumption continues at the projected rate.
While the land reserve is only 14 million tons, the oceanic surface water reserve is estimated to be 230 billion tons based on its average concentration of 1.5 ppm. This supply could last upwards of 7 billion years at the current consumption rate.
This supply can be ethically tapped by utilizing current-activated membranes to produce a concentration of lithium.
Nuclear fission reactors that utilize heavy water as their coolant medium produce a combined 20 kg of tritium annually as a byproduct. Only 8% of reactors use heavy water and this is an incentive for future reactors to be designed as such.
The US generates 2,000 tons of spent fission fuel each year and over 80,000 tons is currently in storage. This waste could be utilized in conjunction with lithium-6 to breed tritium outside of fusion reactors.
The most sustainable method to relieving the tritium bottleneck is the utilization of deuterium-deuterium fusion reactions.
When reacting D-D, tritium and helium-3 atoms are produced. The helium-3 could be further reacted to release tritium and energy, but first generation reactors will likely not be operating at the temperature required for D-He3 fusion.
The primary challenge for D-D reactions will be maintaining stable ignition due to the low energy output. Think of holding a match to a twig; eventually, the twig will ignite and no longer require the input energy of the match.
| Prominent Fusion Reactions | ||
| Fuel Input | Output Product | Energy Release |
| D + D (50%) | Helium-3 | 3.2 MeV |
| D + D (50%) | Tritium | 4.0 MeV |
| D + T | Helium-4 | 17.6 MeV |
| D + He-3 | Helium-4 | 18.3 MeV |
| Li-6 + neutron | Helium-4 + Tritium | 4.8 MeV |
This article identifies deuterium-tritium fuel sources and their extraction methods through the comparison of over a dozen research articles and publications from internationally recognized organizations. It seeks to tie together the fuel to fusion manufacturing chain. Preference is given to publications that obtained data through experimental methods and experience from decades of operation.